[et_pb_section admin_label=”section”] [et_pb_row admin_label=”row”] [et_pb_column type=”4_4″][et_pb_text admin_label=”Text”]
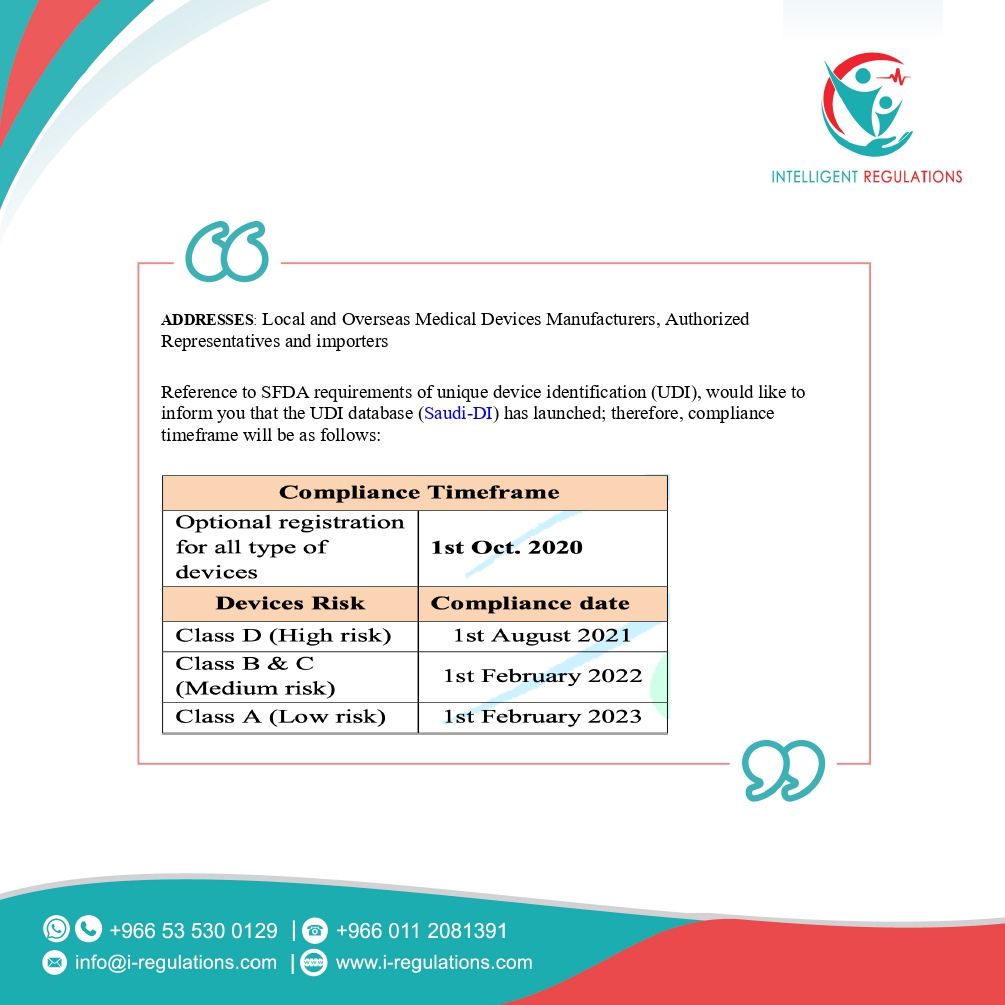
UDI Guidance: https://www.sfda.gov.sa/sites/default/files/2020- 09/MDS-G34e.pdf
UDI database (Saudi-DI): https://udi.sfda.gov.sa
For further enquiries regarding this announcement, please contact
md.rs@sfda.gov.sa or call 19999
[/et_pb_text][/et_pb_column] [/et_pb_row] [/et_pb_section]
