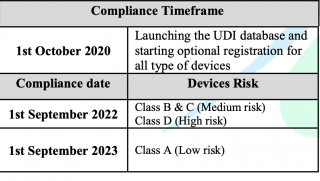
Reference to the published requirements for medical devices unique device identification (Saudi-DI) by Saudi Food & Drug Authority and after launching of UDI database (Saudi-DI), therefore, the timeframe to comply with the requirements has been updated as follows:
- UDI Guidance (MDS-G34): https://sfda.gov.sa/sites/default/files/2020-09/MDS- G34e.pdf
- UDI database (Saudi-DI): https://udi.sfda.gov.sa/
For further inquiries regarding this announcement, please contact md.rs@sfda.gov.sa or call 19999.
