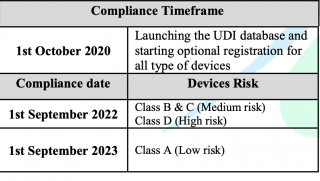
Reference to the published requirements for medical devices unique device identification (Saudi-DI) by Saudi Food & Drug Authority and after launching of UDI database (Saudi-DI), therefore, the timeframe to comply with the requirements has been updated as follows: